

Molecular orbitals are approximate solutions to the Schrödinger equation for the electrons in the electric field of the molecules atomic nuclei. Pi bond: This type of covalent bond is formed by the lateral or sideways overlap of the atomic orbitals. Browse 5,903 sigma photos and images available, or search for sigma bonds or sigma pi to find more great photos and pictures. A molecular orbital (MO) can be used to represent the regions in a molecule where an electron occupying that orbital is likely to be found.
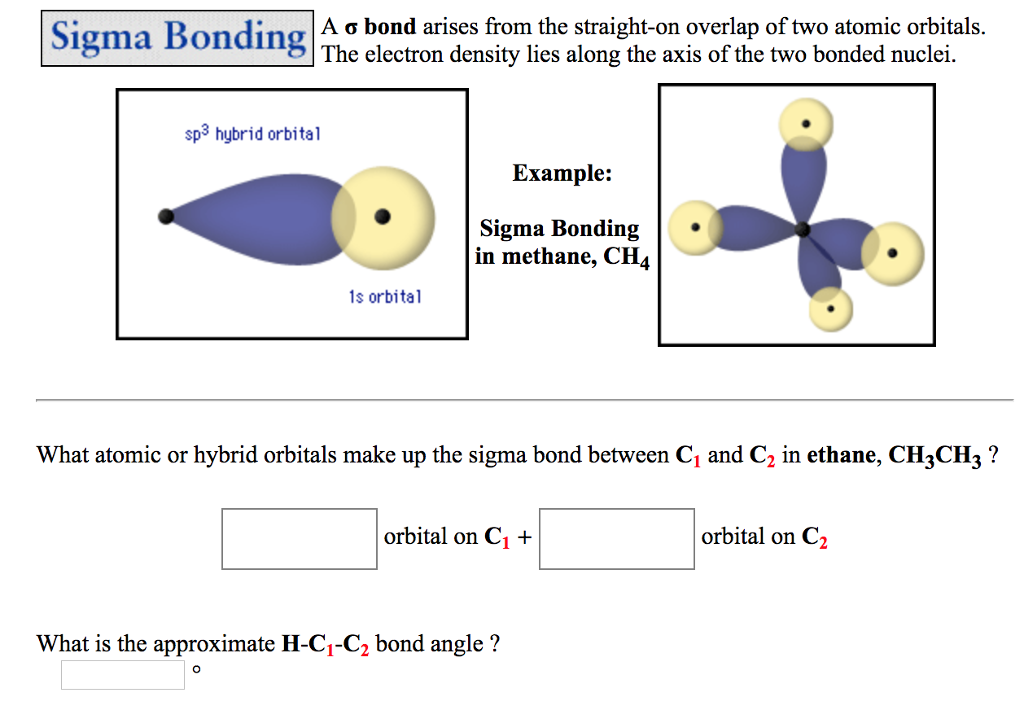
(Bear in mind that the carbons in the methyl group are SP3 hybridised. In the valence bond theory, a sigma bond is a valence bond that is symmetrical. When you have, for example, a methyl group joined on to a double bond, then the electrons in one of sigma bonds joining the C to a H in the methyl group can partially overlap with the electrons in the pi-bond of the double bond. The pi bond ( bond) has two halvesone above the plane of the molecule, and the other below it. The atomic orbitals overlap along the inter-nuclear axis and involve end-to-end or head-on overlap. Most single bonds are sigma bonds (-bond). A second carbon-carbon bond is formed by the overlap of these two remaining p orbitals. \( \newcommand\): Selected examples of \(\sigma\) bonds involving \(d\) orbitals along the \(z\) internuclear axis (shown as a bold horizontal line) between two atoms. Sigma Bond: This type of covalent bond is formed by the axial overlapping of half-filled atomic orbitals.


 0 kommentar(er)
0 kommentar(er)
